Although many of the bands in the IR spectrum can be used to
identify particular functional groups, the region from about 500 - 1500 cm
-1 usually contains a complicated series of bands, many of which overlap. This region contains absorptions which correspond to bending as well as stretching vibrations. It is difficult to pick out individual bands and assign them
reliably to a particular functional group or bond in a molecule. This part of the spectrum is called the
fingerprint region.
Although this region is not very useful for detecting which functional groups are present, it can still be useful for identifying the molecule provided its IR spectrum is already known. Each compound's IR spectrum is unique and so, by matching a spectrum to a library or database either electronically or by eye, it can be positively identified. Such methods are used in forensic, analytical and environmental applications as well as in research chemistry.
The IR spectra of the two isomers of C
4H
10, methylpropane and butane, are shown below. The same types of bonds are present in both molecules and, as a result, the spectra are very similar and the only
characteristic bands present are those due to C-H, which is not particularly useful in identifying which alkane might be present. The differences in the fingerprint region are small but significant enough to be used to positively identify which of these is present at, for example, a suspicious fire.
Note how similar the spectra are about 1500 cm
-1.
Click on the peaks, labelled with red markers, to display the corresponding molecular vibration - these involve the C-H and C-C bonds which are the same in each molecule. It is only below 1500 cm-1 that the spectra differ.
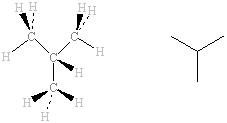
methylpropane |
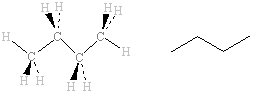
butane |
|
|
|
Some commonly used databases are listed below.